Special EASL-DHILI Consortium-Sponsored Session. 32nd Conference of Clinical Pharmacology, Bratislava (Slovakia)
December 16, 2025, online/onsite
The 32nd Conference of Clinical Pharmacology brought together international experts to discuss current challenges in drug safety, clinical pharmacology, and public health. This year, the EASL‑DHILI Consortium proudly sponsored a dedicated Special Session, reinforcing our commitment to advancing global knowledge on drug‑ and supplement‑induced liver injury.
⭐ Special EASL‑DHILI Consortium‑Sponsored Session: Drug and Herbal & Dietary Supplement‑Induced Liver Injury
Chaired by Maribel Lucena and Helena Glasová, this session highlighted cutting‑edge insights into DILI, with several talks delivered by members of our consortium:
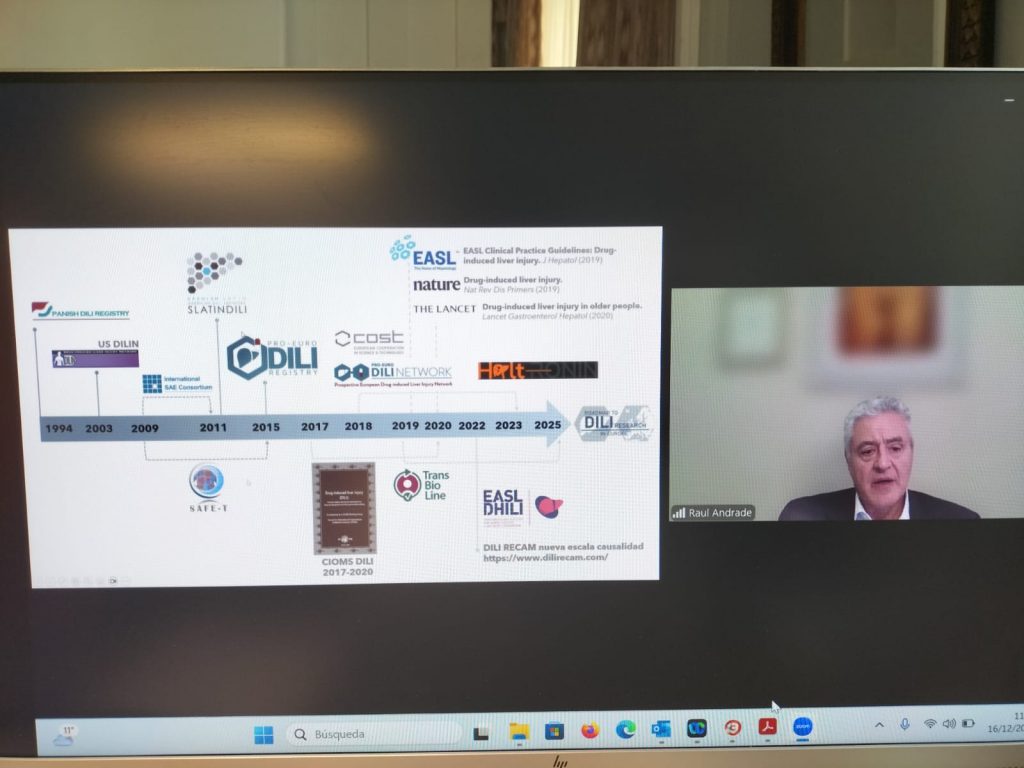
- Raúl J. Andrade (Málaga, Spain) “The contribution of prospective DILI Registries” Showcasing the essential role of structured registries in understanding real‑world DILI patterns and improving patient safety.
- Fernando Bessone (Rosario, Argentina) “Herbal and dietary supplements–induced liver injury: a global challenge” Addressing the rising worldwide impact of supplement‑related hepatotoxicity.
- Einar S. Björnsson (Reykjavik, Iceland) “Liver injury caused by biologics” Providing an expert overview of hepatotoxicity linked to modern biologic therapies.
This session underscored the Consortium’s leadership in DILI research and its global collaborative network.
🔬 Other Scientific Highlights
Beyond the DHILI‑focused session, the conference covered a broad range of clinical pharmacology topics, including:
- Clinical pharmacology in Europe and Slovakia
- Therapeutic drug monitoring in clinical practice
- Pharmacotherapy of cholestatic liver diseases
- Results and impact of the EU‑funded IMAGINE Project on antibiotic use in long‑term care facilities
- A final “Varia” session featuring emerging topics in clinical pharmacology
🤝 Strengthening International Collaboration
The active participation of EASL‑DHILI Consortium members and the sponsorship of a dedicated scientific block reflect our ongoing mission: to improve understanding, diagnosis, and prevention of DILI worldwide through high‑quality research and international cooperation.


She received her PhD in Biochemistry from the Hebrew University. She then joined the laboratory of Prof. Karen Vousden at the Cancer Research UK Beatson Institute in Glasgow, United Kingdom, as a postdoctoral researcher, where she investigated cancer metabolism. Upon returning to Israel as a research scientist at the Faculty of Medicine of the Technion, she shifted her research focus to the metabolic aspects of MASLD. Recently, she established her own laboratory at Ariel University, dedicated to studying how alterations in liver metabolism contribute to the development and progression of MASLD, as well as how these changes affect drug metabolism in the liver.
Dr. Díaz, is an Assistant Professor of the Division of Gastroenterology and Hepatology, University of California San Diego, USA. He was trained as a Gastroenterology and Internal Medicine specialist at the Pontificia Universidad Católica de Chile. As a clinical researcher, he focuses on translational and clinical studies in steatotic liver disease. He has authored more than 160 peer-reviewed publications on gastrointestinal and liver diseases in high-impact journals and co-authored 11 book chapters. He co-leads multinational studies on alcohol-associated liver disease (ALD) and metabolic dysfunction–associated steatotic liver disease (MASLD), including the Global AlcHep Network and STELLA. He serves on the Steering Committee of the ALD Special Interest Group (SIG) of the American Association for the Study of Liver Diseases (AASLD) and chairs the ALD and Public Health SIGs of the Latin American Association for the Study of the Liver (ALEH). Brief Summary: Steatotic liver disease (SLD) represents a heterogeneous spectrum that includes both metabolic dysfunction–associated steatotic liver disease (MASLD) and drug-induced forms, which often overlap in clinical and histologic presentation. Several drugs can induce hepatic steatosis or steatohepatitis through mechanisms involving mitochondrial toxicity, oxidative stress, and altered lipid metabolism. In contrast, patients with MASLD may have increased susceptibility to drug-induced liver injury (DILI), amplifying the risk and severity of hepatotoxicity. Differentiating between metabolic and drug-related causes requires careful evaluation of medication history, temporal associations, and exclusion of other etiologies, as both conditions may coexist and interact. In this talk, we discussed the most relevant features of both SLD subtypes in clinical practice.

«Pharmacogenomics in DILI: a pathway for personalized precision medicine».Prof. Ann Daly, Translational and Clinical Research Institute, Faculty of Medical Sciences, Newcastle University, Newcastle, UK.
«Design, endpoints and challenges in conducting randomized clinical trials in DILI». Prof. Yimin Mao, Division of Gastroenterology and Hepatology, Shanghai Institute of Digestive Diseases, NHC Key Laboratory of Digestive Diseases, Shanghai Research Center of Fatty Liver Disease, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
EASLDHILI & IUPHAR Session B7: Drug-induced liver injury – Current topics
Monday, 30th June 2025 * 17.00h-18.30 (EET)
Chairs: Caroline Samer, University of Geneva, Geneva, Switzerland and M Isabel Lucena, University of Malaga, Malaga, Spain

Dr. Isabel García Martín. Her research career began with a PhD in Chemistry (2003, Universidad Autónoma de Madrid), focusing on natural product synthesis at CSIC-IQO. After postdoctoral work in supramolecular chemistry, she joined the group of Prof. S. Penadés to explore gold nanoparticles functionalized with biomolecules for biomedical applications. This marked the beginning of her multidisciplinary path, integrating chemistry, materials science, and biology. Since then, she has developed smart nanotechnology-based systems for biomedical imaging, diagnostics, and therapy as part of the CIBER-BBN network at CIC biomaGUNE. Currently based in the BioNanoplasmonics Lab (LNB-CICBIO, Prof. Luis M. Liz-Marzán), she specializes in the design of gold-based nanostructures for targeted imaging, nanovaccines, and biosensing. She has contributed to multiple collaborative national projects involving innovative nanocarriers, skin-on-chip models, and theranostic tools, and has co-led projects funded by ISCIII and AECC, maintaining close ties with clinical partners and biotech companies. Nanotechnology is transforming multiple disciplines, especially healthcare, by enabling highly precise diagnostic and therapeutic strategies. In the realm of diagnostics, nanoscale technologies provide exceptional sensitivity and specificity through innovations such as nanoparticle-based assays, biomarker detection platforms, and point-of-care devices. These breakthroughs support early disease identification and the advancement of personalized medicine, leading to better patient outcomes. When integrated with imaging modalities and as drug carrier, nanotechnologies enhance the visualization of molecular processes, improving both diagnostic accuracy and disease monitoring even after treatment. In the seminar, I will feature several technological nanoplatforms developed throughout my carrier that provide values biological information in different biological scenarios of interest. Most recently, we have been working on targeted nanotherapies conjugated to a specific aptamer that specifically block HuR SUMOylation over-expressed in hepatic cancer, with the goal of improving the prognosis of liver cancer. In this project, we have developed surfaces that mimic the orientation of the hepatic asialoglycoprotein receptor (ASGPR receptor) to evaluate, in vitro, the targeting efficiency of our nanotechnology-based delivery systems.

Magnus Ingelman-Sundberg, PhD; BSc.Med is Professor of Molecular Toxicology and research group leader in Pharmacogenetics at the Department of Physiology and Pharmacology, Karolinska Institutet. He has more than 520 original papers and a h-factor of 101 (ISI/Clarivate) or 132 (Google Scholar). Assigned “Highly Cited Researcher” for 2014-2017 and 2021-2024 by Thomson & Reuters/Clarivate. His research focuses on genetics, polymorphism, regulation, function and toxicology of the hepatic ADME system with aims at understanding interindividual differences in drug response. Furthermore he develops novel hepatic in vitro systems for studying liver function, liver diseases and validation of hepatic drug targets. Summary The lecture will be focused on our development of a 3D liver spheroid system that closely mirrors the transcriptome, proteome, and metabolome of the corresponding donor liver. This model has proven highly effective in: i) predicting and understanding mechanisms behind drug-induced hepatotoxicity, ii) assessing the hepatic disposition and metabolite formation of low-clearance drugs, iii) elucidating the mechanisms of viral-induced hepatitis and the specific actions of viruses within the liver, iv) evaluating mechanisms of hepatocyte proliferation and v) understanding the processes by which a high-fat diet induces liver fibrosis and the molecular drivers of liver fibrosis degradation.

Las XXIV Jornadas de Avances en Hepatología se celebrarón los pasados días 15 y 16 de Mayo de 2025 en el Aula Magna de la Facultad de Medicina de la Universidad de Málaga.
Durante estas jornadas se discutiron numerosos temas de actualidad e interés en Hepatología que serán impartidos por profesionales con reconocimiento internacional en la materia. Estas jornadas brindan posibilidades de encuentro y establecimiento de colaboración en una atmosfera distendida y cordial y en un marco privilegiado, que es el que ofrece nuestra ciudad particularmente en esta época del año. Las Jornadas de Hepatología de Málaga son ya un curso de referencia en el panorama nacional.
El programa se estructura en torno a 5 mesas redondas y una conferencia especial impartida por la Dr. Miguel Angel Martínez de la Universidad de Navarra, Pamplona; Harvard T.H. Chan School of Public Health, Boston, MA, USA, experto mundial en Salud pública y en dieta mediterránea en la prevención de enfermedades.
Además, el curso está acreditado por la Dirección General de Investigación y Gestión del Conocimiento de la Consejería de Salud de la Junta de Andalucía. También tiene validez como Actividad Formativa de Doctorado, 2 créditos (50 horas). Programa Doctorado BIOMEDICINA, INVESTIGACIÓN TRASLACIONAL Y NUEVAS TECNOLOGÍAS EN SALUD, Facultad de Medicina, Universidad de Málaga.

IAIHG (International Autoimmune Hepatitis Group) Meeting @EASL Congress 2025
Wednesday 7 May. 1.30pm-2.30pm 13:30-14:30 (CET). Room D201 (Level 2 of Elicium building).
Our DHILI Consortium in collaboration with EASL chaired & co-organized a General Hepatology Session @EASL Congress 2025. 9 May 2025
EASL DHILI Do’s and don’ts in diagnosis and management of drug-induced liver injury
3 EASL DHILI Consortium meeting
@EASL Liver Congress 2025. 9 May 2025
Amsterdam, The Netherlands

Volker M. Lauschke is Professor in Translational Pharmacology at Karolinska Institutet, Stockholm and Deputy Head of the Bosch Institute of Clinical Pharmacology in Stuttgart, Germany. In addition, he holds Guest Professorships at the Second Xiangya Hospital, Central South University and at Lanzhou University, China. Besides his academic work, he is co-founder and CEO of HepaPredict AB as well as co-founder of Shanghai Hepo Biotechnology Ltd, which provide innovative 3D human tissue cultures for drug discovery and development.
In this talk, he presented an overview of how 3D cell culture systems of primary human cells and microfluidics can be integrated with phenotypic and chemogenomic screening to identify novel targets for steatotic liver disease.

Bernard Fromenty is a research director at INSERM and currently co-directs one of the teams at the NUMECAN Institute in Rennes, France. He has been working for 35 years on liver damage induced by different xenobiotics including drugs, alcohol and environmental toxins. His research aims to determine the mechanisms by which certain xenobiotics can induce different liver lesions with a particular interest for steatosis and steatohepatitis. More specifically, his research focuses on mitochondrial dysfunction, alteration of lipid homeostasis and oxidative stress. More recently, he has extended his research to the issue of hepatotoxicity in the context of obesity and MASLD. He is the co-author of more than 140 original articles and reviews.

Patients with underlying disease when they develop DILI are at significantly higher risk for worse outcomes such as liver failure, liver transplantation, or liver related death. However, it remains unclear if underlying liver disease is a risk factor for developing DILI. Historically, underlying liver disease, especially cirrhosis has been stated as a risk factor for DILI from isoniazid. Similarly, compounds such as methotrexate and tamoxifen may be more hepatotoxic in individuals with underlying chronic liver disease. In this lecture, the speaker will discuss the literature on the relationship between underlying liver disease and the risk for developing idiosyncratic DILI.
His presentation identifies hepatic mitochondrial derived 27-hydroxycholesterol, an oxysterol whose generation is controlled by STARD1-dependent cholesterol delivery to mitochondrial inner membrane, as a previously unrecognized link between MASH development and cardiovascular disease (CVD). Although MASH is an advanced chronic liver disease of high prevalence due to its association with the obesity pandemic, CVD is one of the leading causes of mortality among MASH patients. Targeting hepatic STARD1 or 27-hydroxycholesterol may offer a novel therapeutic opportunity for MASH-driven CVD.

Águeda González Rodríguez obtained the PhD in Pharmacy (2008) at Complutense University of Madrid (Madrid, Spain). She had her postdoctoral training (2008-2014) at the Sols Morreale Biomedical Research Institute (IIBM, Madrid, Spain), a joint center run by Spain’s Higher Science Research Council (CSIC) and the Autonomous University in Madrid, and at the University of Cambridge (Cambridge, UK). From 2015, she heads the Metabolic Syndrome and Vascular Risk research group, firstly at the Health Research Institute Princesa (Madrid, Spain) and, since 2022, at IIBM (Madrid, Spain) as CSIC Senior Scientist, developing a translational “bench to bed/bed to bench” research focused on the study of the molecular basis of Metabolic Syndrome (MetS) and its comorbidities, with a major focus on metabolic-associated steatotic liver disease (MASLD). Moreover, her group studies molecular mechanisms involved in the progression of acute and chronic liver injury, including drug induced hepatotoxicity. In this regard, her team are investigating the role of bone morphogenetic proteins (BMPs) in the progression of acetaminophen (APAP)-induced acute liver failure (ALF). Indeed, her group was the first to identify the protective effect of ALK3 receptor inhibition against APAP-induced hepatotoxicity, suggesting that BMP signalling might be a new pharmacological target for the treatment of ALF. Current research efforts include the search of new serological biomarkers based on BMPs for prognosis of ALF.

Graduated in Biology in 1993 and PhD in Biochemistry and Molecular Biology by the UAM (Madrid, Spain) in 1997. Following a postdoctoral stay at the Dana Farber Cancer Institute in Boston (MA, USA), returned to Spain with a Ramón y Cajal contract that allowed her to develop her own research line first within the group of Santiago Lamas at CIB, CSIC (Madrid, Spain) and then as independent group leader at CNIC. From 2011 she joined the IIBM, CSIC-UAM first as Científico Titular CSIC and then as Investigador Científico CSIC where she leads the Mitochondrial Function in Health and Disease group. She is currently Council Member of the SEBBM, Vicedirector of GEIRLI, member of SED and e-mit as well as the COST action CA20121.
Liver steatosis is a highly prevalent condition associated with the metabolic syndrome that can progress to metabolic associated fatty liver disease (MAFLD) and is a mayor risk factor for hepatocarcinoma (HVV) development. MAFLD is characterized by the presence of fibrosis, inflammation as well as mitochondrial dysfunction and oxidative stress. However, the contribution of mitochondria to disease progression and HCC development remains to be stablished. To that address this question we studied the effect of high fat diet and teratogen administration on a mouse model of mitochondrial dysfunction, deficient in the protein PGC-1α, a master regulator of mitochondrial function. Our data suggest that PGC-1 α deficiency facilitates HCC development and this effect is likely related to a deficient capacity to respond to cellular damage, highlighting the critical role played by mitochondria in the cellular response to stress and its impact on HCC.

Leo van Grunsven obtained his Biology degree in 1992 from the University of Utrecht and obtained his PhD in 1996 from the Ecole Normale Supérieure de Lyon (France). He had his postdoctoral training at the NINDS/NIH (Bethesda, USA) and the KU Leuven (Belgium) and joined the lab of the late Prof. Albert Geerts at the Vrije Universiteit Brussel (VUB, Belgium) in 2006, and became an assistant Professor in 2009 and heads the Liver Cell Biology research group since.
His group studies molecular mechanisms involved in liver -homeostasis, -fibrosis and –regeneration with a special focus on hepatic stellate cells. His group was the first to identify autophagy, AGE- and HIPPO-signaling as key mechanisms involved in hepatic stellate cell activation during liver fibrogenesis. His team established the first hepatocyte-injury dependent in vitro liver fibrosis model by using spheroid cultures of human hepatocytes and hepatic stellate cells (2016). Current research efforts include the development of more advanced in vitro systems for chronic liver disease using primary mouse and iPSC-derived liver cells, and investigation of stress pathways in hepatic stellate cells and other sinusoidal liver cells during acute and chronic liver injury.

EASL Congress 2024 has now ended!
Bringing together over 7,000 professionals, including clinicians, researchers, allied health professionals, patient representatives, and industry professionals, the EASL Congress stands out as Europe’s largest event in this domain.
Four days of congress full of interactivity and hands-on learning sessions from world-class faculty. Excellent opportunities for the liver community to present their research, network and interact face-to-face with the greatest minds in hepatology.
Our DHILI Consortium in collaboration with EASL chaired & co-organized a General Hepatology Session @EASL Congress 2024
Unpacking Drug-Induced Liver Injury Challenges and Research Horizons




2º EASL DHILI Consortium meeting @EASL Liver Congress 2024
Milan, Italy.
SLIDES AVAILABLE HERE



Gerd A. Kullak-Ublick, M.D. has a dual appointment as Full Professor of Clinical Pharmacology and Toxicology at the University Hospital Zurich, University of Zurich/Switzerland, and as Global Head of Mechanistic Safety and Safety Signal Management at Novartis, Basel/Switzerland. Gerd also co-leads the DILI work package in the IMI TransBioLine Consortium. Gerd is board-certified in Internal Medicine, Gastroenterology/Hepatology and Clinical Pharmacology/Toxicology. His research interests are centered on drug transporters in epithelial tissues (liver, intestine, kidney, blood-bran barrier), and on mechanisms of drug-induced liver injury. Gerd is a member of the Pro-Euro DILI Consortium, the IQ DILI Consortium and the CIOMS DILI Working Group.
Drug-induced liver injury (DILI) is characterized by increased levels of biochemical markers of liver injury within days to months after the start of pharmacological treatment for any given condition. Often, patients receive more than one medication, or have underlying liver conditions which can confound the interpretation of liver laboratory testing. In clinical trials, the decision as to whether or not to interrupt treatment with a study drug is based on algorithms defined in regulatory guidances that use standard liver lab testing values – ALT, AST, bilirubin, and alkaline phosphatase. There is a need for more specific markers of liver injury that could improve the management of DILI. The IMI TransBioLine Consortium is focused on the evaluation and validation of novel liver safety biomarkers for DILI. The Consortium was issued a Letter of Determination by the FDA in 2021 that encouraged the further assessment of HMGB1 (high mobility group box 1), K18 (cytokeratin 18 full-length), cc-K18 (cytokeratin 18 – caspase-cleaved fragment), GLDH (glutamate dehydrogenase), OPN (osteopontin), MCSF1R (macrophage colony stimulating factor receptor 1), bile acids and sphingolipids. The Consortium has recruited more than 100 DILI samples in the exploratory phase which have undergone analysis for the above named biomarkers. Based on these results, a Biomarker Qualification Plan (BQP) is currently in preparation which will detail the exact Context-of-Use for each biomarker and the pre-defined endpoints that need to be met so as to fulfill regulatory requirements for qualification.
Dr. Pau Sancho-Bru was our last speaker. He is Group Leader of the Liver Cell Plasticity and Tissue Repair group at IDIBAPS, and Associate Professor at University of Barcelona. His group is conducting translational research in the field of liver diseases, investigating the role of cell plasticity in wound healing and carcinogenesis. One of the main research interests of his group is assessing the potential of stem cells for biomedical and biotechnological applications and particularly to develop 3D organotypic in vitro systems for disease modeling and drug development.
Liver Organoides for steatotic liver disease modelling: a new venue of opportunities
The talk introduced the different technologies available to generate patient derived liver organoids and discuss their potential to study liver disease and drug development. It will also show the work being performed in the lab regarding the generation of patient-derived liver organoids from liver needle biopsies. Moreover, the talk will present the results we have obtained showing the potential of these organoids to reproduce patient-specific characteristics and the epithelial compartment of the liver.

Prof. Malú Martínez-Chantar is an experienced liver biology and disease researcher with a strong publication record in top journals. She has received continuous support from national and international public and private funding, including the NIH. Prof. Martinez-Chantar coordinates the Translational Area at the National Institute for the Study of Liver & Gastrointestinal Diseases and is involved in the Scientific Advisory Boards of national and international centers. She actively participates in various networks such as CibereHD, Women in Hepatology: International Consortium, Hepamet Registry, MetaboCancer Excellence Network, and EU COST actions. Professor Martinez-Chantar’s collaborative efforts with pharmaceutical companies have culminated in numerous patent applications and the successful licensing of products. In a recent entrepreneurial endeavor, she established a spin-off company, Gibela Therapeutics, based in Basel, Switzerland, with a focus on advancing treatments for liver diseases. This presentation unfolds at the intersection of pioneering research and innovative solutions in hepatic diseases. «Translational Basic Research: Navigating Opportunities and Challenges in the Development of Therapeutic RNA Molecules for Addressing Hepatic Diseases via Magnesium Homeostasis» explores a transformative approach to hepatic conditions by leveraging the nuanced dynamics of magnesium homeostasis.
The session aims to navigate through the promising opportunities that therapeutic RNA molecules present in reshaping the landscape of hepatic diseases. By delving into the profound impact of magnesium homeostasis, attendees will gain insights into the pivotal role it plays in the development and application of therapeutic interventions. Together, we aspire to pave the way for groundbreaking advancements in hepatic healthcare.

Sarp Uzun was born in Adana, Turkey. He graduated from Hacettepe University Faculty of Medicine in 2020 in Ankara, Turkey. In 2016, he enrolled in the MD-PhD program at the same university and received his PhD degree in 2021 from the Tumor Biology and Immunology Ph.D. program. During his PhD, he worked on β-Catenin N-terminal region alterations in neoplastic diseases. He joined the lab of PD.Dr. Matthias Matter at the Institute of Pathology, University Hospital Basel in August 2021. Currently, he is working on molecular and morphological analysis of immune-mediated liver diseases, primarily SARS-CoV-2 vaccine-induced liver injury and checkpoint-inhibitor-induced liver injury.
Our first speaker was Prof Jonathan Fallowfield, personal chair of Translational Liver Research at the University of Edinburgh and Honorary Consultant Hepatologist at the Royal Infirmary of Edinburgh (UK).
Professor Jonathan Fallowfield’s research interests span basic science and translational/clinical studies in hepatology. Key topics include mechanisms of liver fibrogenesis and fibrosis regression; portal hypertension and hepatorenal syndrome; biomarkers (particularly imaging); and discovery/development of novel therapies for liver fibrosis, NASH and portal hypertension.
4th Swiss Autoimmune Liver Disease Meeting in Lugano, Switzerland, September 21-22, 2023
Fondazione Epatocentro Ticino hosts its 4th Swiss Autoimmune Liver Disease Meeting in Lugano, Switzerland, on September 21-22, 2023.
The conference is bringing together world’s top experts in the field of autoimmune liver diseases in collaboration with members of the EASL DHILI Consortium). Learn more about speakers and program here.


EASL DHILI Consortium meeting @EASL Liver Congress 2023. Vienna, Austria. 21-24 June 2023.
Our first official meeting took place during the EASL Congress in Vienna on June 23rd 2023 between 11.00 and 12.00 pm (CEST time) in the Schubert room 4.
The main objective was to discuss opportunities this consortium brings and set goals for the next 6 months.
Drug-Induced Liver Injury (DILI) Diagnosis: More Than Guilt by Association
19 June 2023
The webinar has been focused on the identification, diagnosis of drug, and herb-induced liver injury. Each topic has been chosen based on a key publication that addresses an important challenge in drug-induced liver injury.


Las XXII Jornadas de Avances en Hepatología se celebrarón los pasados días 18 y 19 de Mayo de 2023 en el Aula Magna de la Facultad de Medicina de la Universidad de Málaga.
Durante estas jornadas se discutieron numerosos temas de actualidad e interés en Hepatología impartidos por profesionales con reconocimiento internacional en la materia. Estas jornadas brindaron posibilidades de encuentro y establecimiento de colaboración en una atmosfera distendida y cordial y en un marco privilegiado, que es el que ofrece nuestra ciudad particularmente en esta época del año. Las Jornadas de Hepatología de Málaga son ya un curso de referencia en el panorama nacional.
El programa se estructuró en torno a 6 mesas redondas, una charla-coloquio y una conferencia de clausura impartida por el Dr. Pere Ginés, del Hospital Clínic de Barcelona; experto mundial en enfermedades hepáticas y en particular cirrosis hepática.
Además, el curso está acreditado por la Dirección General de Investigación y Gestión del Conocimiento de la Consejería de Salud de la Junta de Andalucia. También tiene el reconomiento de interés sanitario-científico por la Junta de Andalucía. También tiene validez como Actividad Formativa de Doctorado, 1 crédito (25 horas). Programa Doctorado BIOMEDICINA, INVESTIGACIÓN TRASLACIONAL Y NUEVAS TECNOLOGÍAS EN SALUD, Facultad de Medicina, Universidad de Málaga.
Sede: Aula Magna, Facultad de Medicina, Universidad de Málaga (Boulevard Louis Pasteur, 32. Campus de Teatinos, S/N, 29010 Málaga)
Organizadores: Dpto de medicina y Unidad de Gestión Clínica de Digestivo; Instituto de Investigación Biomédica de Málaga (IBIMA); Hospital Universitario Virgen de la Victoria. Facultad de Medicina, Universidad de Málaga; Prospective European Drug-Induced Liver Injury.